How to Tell Which Element Has the Lowest Ionization Energy
This means the electrons have the lowest possible values for n the principal quantum number. When multiple orbitals of the same energy are available the lowest energy state is the one with a single electron in an orbital.
Ionization Energy Trends Of The Periodic Table
The lowest ionization energy of any element is 389 eV for caesium.

. Lithium metal has a first ionization energy work function of 520 Kjmol. Academiaedu is a platform for academics to share research papers. Francium element number 87 at the bottom of the first period has a very low electronegativity while fluorine element number 9 at the top of period 17 has a very high.
Electron B feels more effective nuclear charge Zeff than electron A. The ground state is the state in which the electrons in the atom are in their lowest energy levels possible atoms naturally are in the ground state. A H and H 2 b N and N 2 c O and O 2.
Allowing for a few exceptions electronegativity increases as you move up and toward the right. Compare the atomic and molecular orbital diagrams to identify the member of each of the following pairs that has the highest first ionization energy the most tightly bound electron in the gas phase. Ionization potential refers to the energy required as input to an atom to remove the first valence electron ie a measure of how tightly bound electrons are to the atom and the electron.
Algebra I Module 2. It tends to decrease down a column of the periodic table because the number of electron shells is larger making each ion further away. What is the most common oxidation.
On the periodic table as atoms increase in size the amount of energy needed to remove an electron decreases. When electrons occupy the same orbital there is a slight repulsive and destabilizing interaction. Ionization energy is the amount of energy necessary to remove an electron from an atom.
A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same. A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO method in particular. Which element has the lowest first ionization energy.
Ionization energy is the amount of energy needed to remove an electron from an atom. The tendency of an atom toward a configuration in which it possesses eight valence electrons is referred to as the Octet Rule There are two ways for an atom that does not have an octet of valence electrons to obtain an octet in its outer shell. Electron B has more energy than electron A.
One way is the transfer of electrons. Write the charge that each of the following atoms will have when it has a complete set of valence electrons forming an ion. If light with a wavelength of 300 nm is shined upon a piece of lithium metal at.
For the first ionization energy for an N 2 molecule what molecular orbital is the electron removed from. O 2- Na 1 F 1- N 3- Ca 2 Ar - none 3. You can determine relative electronegativity by looking at the periodic table.
Cesium as the largest atom the lowest ionization energy and the most reactivity with nonmetals. Thus it is. Roughly this corresponds to both the first ionization energy of oxygen and the ionization energy of hydrogen both about 14 eV.
See diagrams above for neon for example It is this spectral fingerprint that astronomers use to identify the presence of the various chemical elements in. The ionization energy tends to increase from left to right across the periodic table because of the increase number of protons in the nucleus of the atom. In these lower energy states the outermost energy level has eight electrons an octet.
Specific quantized amounts of energy are needed to excite an electron in an atom and produce an excited state. In this module students reconnect with and deepen their understanding of statistics and probability concepts first introduced in Grades 6 7 and 8. This can be determined by its position lowest in the alkali metal group.
Extending this a bit it should become clear that since every chemical element has its own unique set of allowed energy levels each element also has its own distinctive pattern of spectral absorption and emission lines. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. Element D has an energy level I of 2 e negative an energy level II of 8 e negative and an energy level III of 1 e negative.
In some Environmental Protection Agency references the ionization of a typical water molecule at an energy of 33 eV is referenced as the appropriate biological threshold for. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. Nitrogen has all three 2p orbitals singly occupied and therefore the next electron which corresponds to oxygen has to pair up in one of the p orbitals.
What Is The Difference Between The Ionization Energy Of Na And Mg Quora

The Parts Of The Periodic Table

Solved Element Has The Lowest Ionization Energy

Periodic Trends In Ionization Energy Ck 12 Foundation
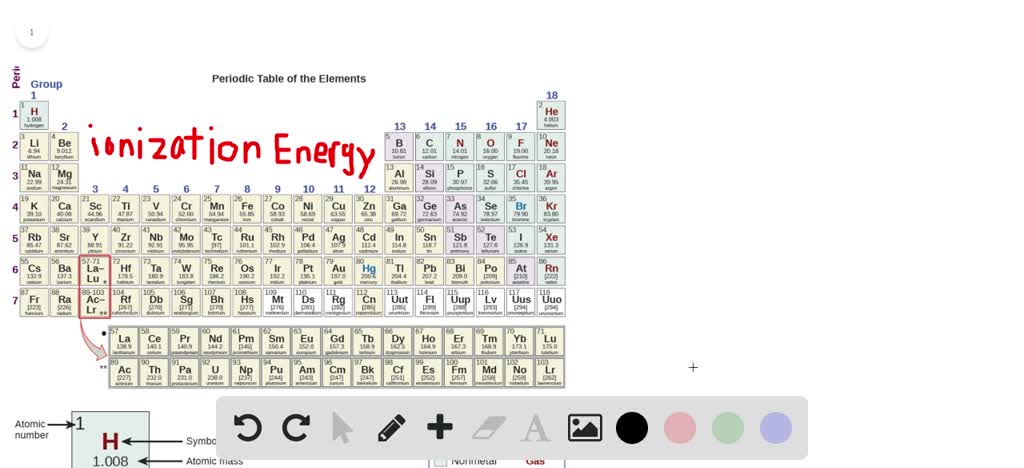
Solved Based On Their Positions In The Periodic Table Predict Which Has The Smallest First Ionization Energy Li Cs N F I
What Are The Periodic Trends For Atomic Radii Ionization Energy And Electron Affinity Socratic
Ionization Energy And Electron Affinity
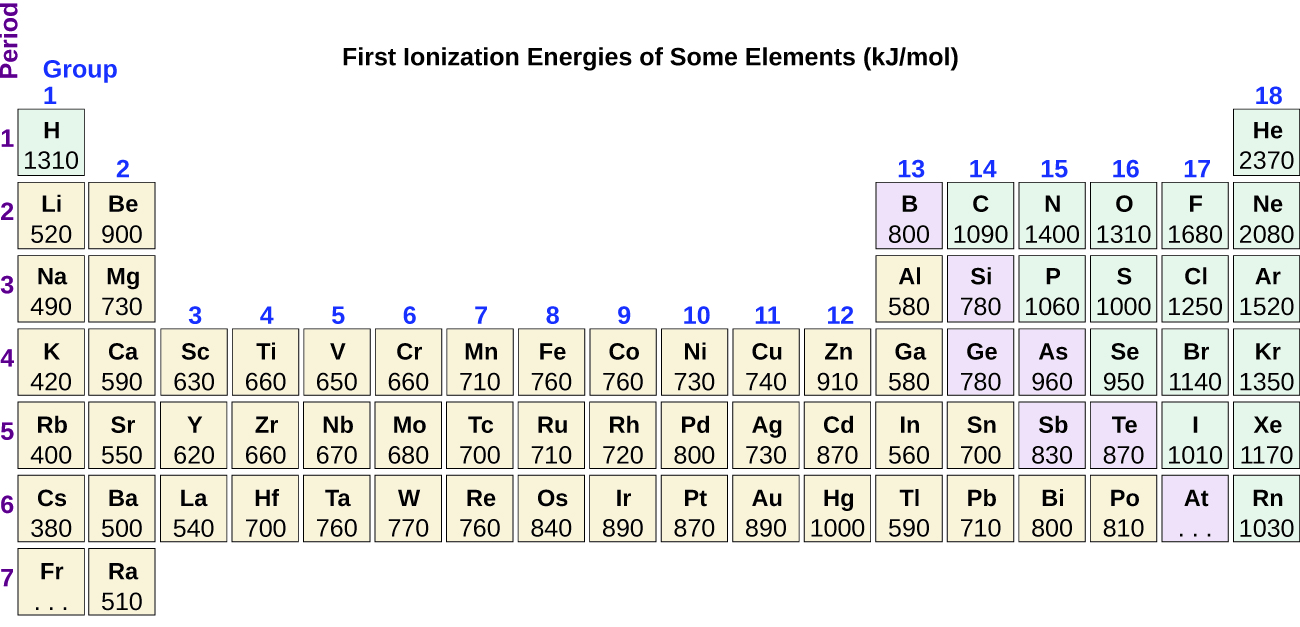
6 5 Periodic Variations In Element Properties Chemistry

Which One Has The Lowest First Ionization Energy Ca P Or Ci Quora

Metallic Character Trend On The Periodic Table
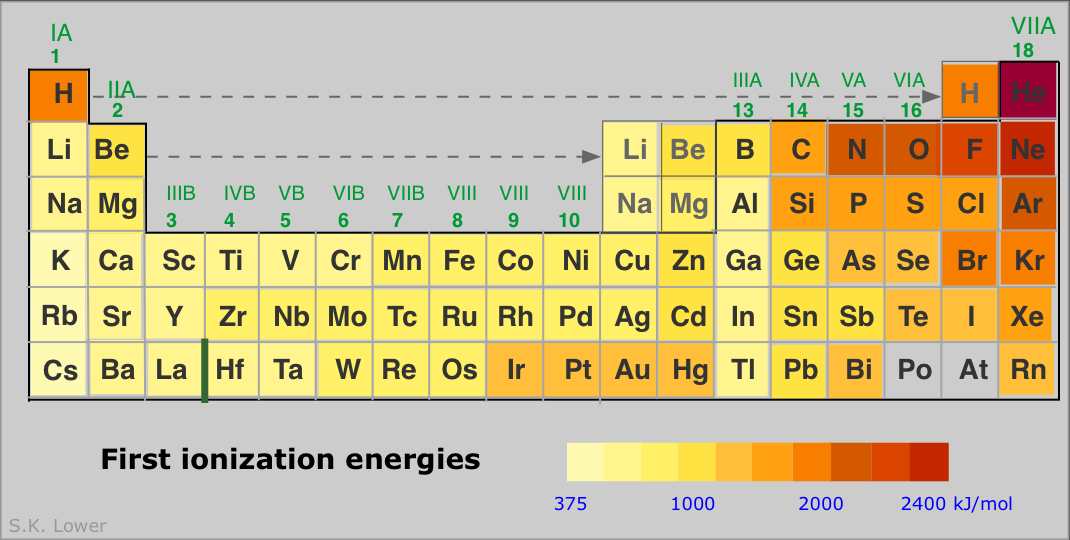
2 10 Periodic Properties Of The Elements Chemistry Libretexts
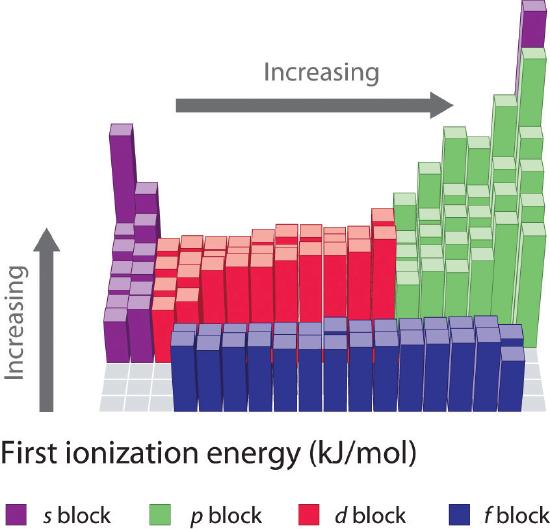
7 4 Ionization Energy Chemistry Libretexts

The Parts Of The Periodic Table

Electron Affinity Of The Elements
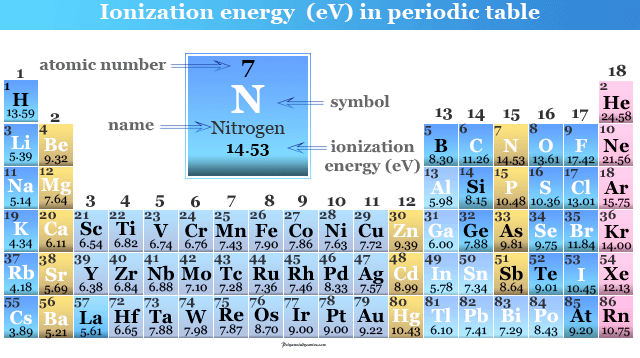
Ionization Energy Definition Equation Periodic Table Trends

Inorganic Chemistry How Can I Relate The Reactivity Series To Electronegativity And Ionization Energy Chemistry Stack Exchange
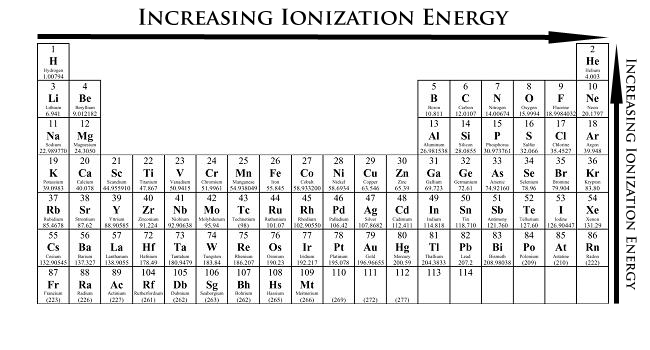
How Would You Arrange The Following Elements In Order Of Increasing Ionization Energy Te Pb Cl S Sn Socratic
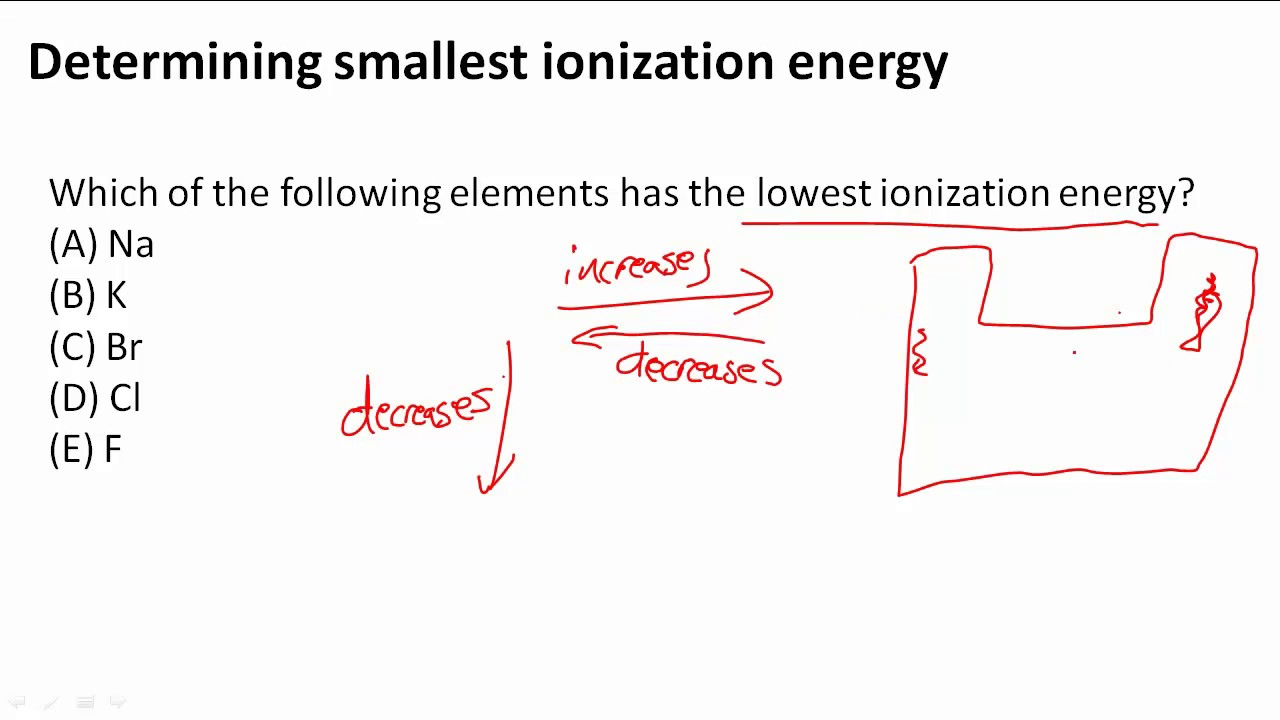
Comments
Post a Comment